Post Market Clinical Follow-up
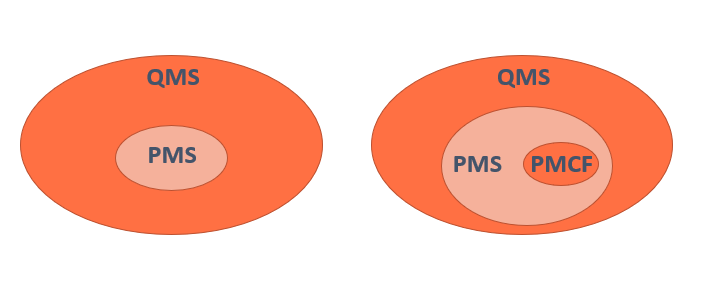
El MDR ha introducido nuevos requisitos para el Post Market Clinical Follow-up (PMCF). Introducción Según el MDR, el Post Market Clinical Follow-up (PMCF) será entendido como un proceso continuo, y cuya finalidad es realimentar a otros procesos con el único objetivo de mejorar la seguridad y el desempeño de los productos sanitarios durante todo su … Leer más